Single-molecule localization microscopy methods, such as STORM and DNA-PAINT, belong to the most widely used techniques of fluorescence nanoscopy because they provide very good spatial resolution with a simple experimental set-up. STORM reaches lateral resolutions < 20 nm, and DNA-PAINT < 10 nm. However, achieving the same level of resolution in the axial direction of the microscope is quite more challenging, and until now was only achieved with highly sophisticated methods such as 4Pi-STORM or MINFLUX.
In our recent paper in Nature Communications, we present a calibration and analysis method for SMLM data obtained under total internal reflection fluorescence (TIRF) conditions, that retrieves the axial position of the single-molecules from its intensity. The new method, called SIMPLER (Supercritical Illumination Microscopy Photometric z-Localization with Enhanced Resolution), is so precise that delivers nearly isotropic resolution in 3D for any SMLM method. For example, in combination with DNA-PAINT, SIMPLER allows the visualization of the cross-section of individual microtubules with great detail!
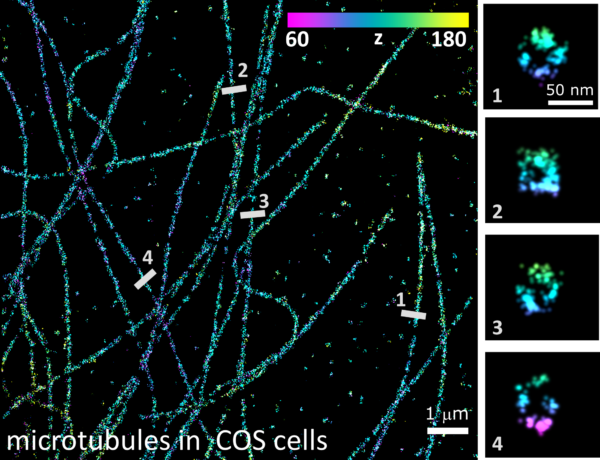
The great advantage of SIMPLER is that can be applied to any SMLM data obtained on any TIRF microscope with no hardware modification whatsoever. The drawback is the limited axial field of view of TIRF measurements. In the publication, we also provide calibration parameters for the most usual microscope configurations, application software, and example data so that any interested microscopist can start using it right away.
Nature Communications 12 (2021) 517
“Three-dimensional total-internal reflection fluorescence nanoscopy with nanometric axial resolution by photometric localization of single molecules”
Alan Szalai, Bruno Siarry, Jerónimo Lukin, David J. Williamson, Nicolás Unsain, Alfredo Cáceres, Mauricio Pilo-Pais, Guillermo Acuna, Damián Refojo, Dylan M. Owen, Sabrina Simoncelli, Fernando D. Stefani



