The protein kinase A (PKA) is an evolutionarily conserved regulator of diverse cellular processes, so advances in understanding its function are impactful.
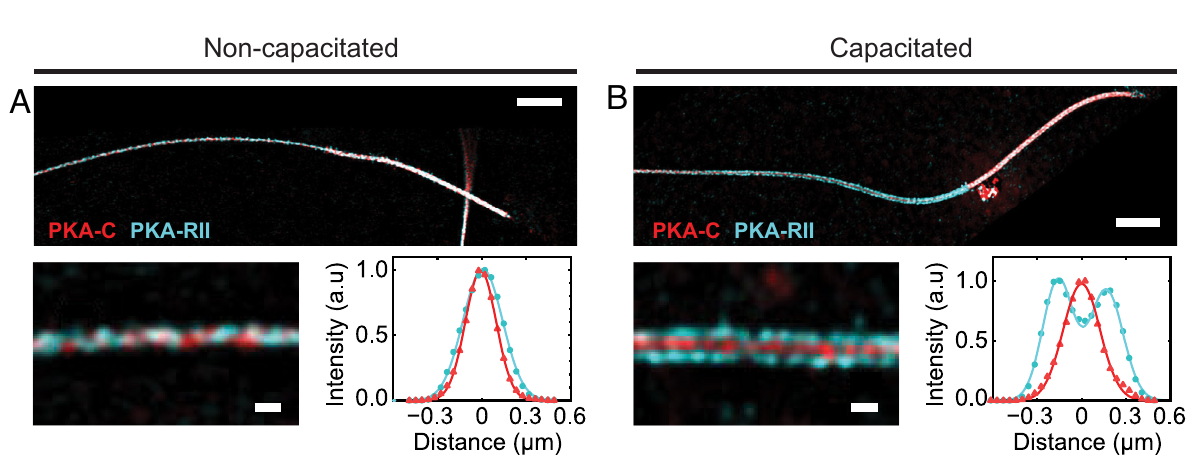
In mammalian sperm, PKA modulates motility and plays a critical role in capacitation, a postejaculation maturation step that enables sperm to fertilize an oocyte. This process depends on PKA regulatory subunits keeping PKA inactive until activated by cyclic AMP (cAMP) and on its intracellular localization. Yet, the localization and interaction of PKA subunits remain poorly understood.
In this collaborative work led by the group of Darío Krapf at the Instituto de Biología Molecular y Celular de Rosario (IBR) we reveal a dramatic subdiffraction relocalization of PKA subunits and demonstrate how scaffolding proteins modulate PKA during capacitation. Furthermore, regulatory subunits of PKA that serve as a biomarker for sperm capacitation are identified, addressing a long-standing challenge in developmental biology research.
Analia G. Novero, Arturo Matamoros-Volante, Lucila R. Gomez-Olivieri, Cintia Stival, Guillermina M. Luque, Alan M. Szalai, Andrea L. Ambrosio, Fernando D. Stefani, Mariano G. Buffone, Dario Krapf, Diego Krapf