Fluorescence Resonance Energy Transfer (FRET)-based approaches are unique tools for sensing the immediate surroundings and interactions of (bio)molecules.
FRET imaging and Fluorescence Lifetime Imaging Microscopy (FLIM) enable the visualization of the spatial distribution of molecular interactions and functional states. However, conventional FLIM and FRET imaging provide average information over an ensemble of molecules within a diffraction-limited volume, which limits the spatial information, accuracy, and dynamic range of the observed signals.
In our recent publication in collaboration with the group of Prof. Guillermo Acuna at the University of Fribourg (Switzerland), we present an approach to obtain super-resolved FRET imaging based on single-molecule localization microscopy using an early prototype of a commercial time-resolved confocal microscope is demonstrated. DNA Points Accumulation for Imaging in Nanoscale Topography with fluorogenic probes provides a suitable combination of background reduction and binding kinetics compatible with the scanning speed of usual confocal microscopes. A single laser is used to excite the donor, a broad detection band is employed to retrieve both donor and acceptor emission, and FRET events are detected from lifetime information.

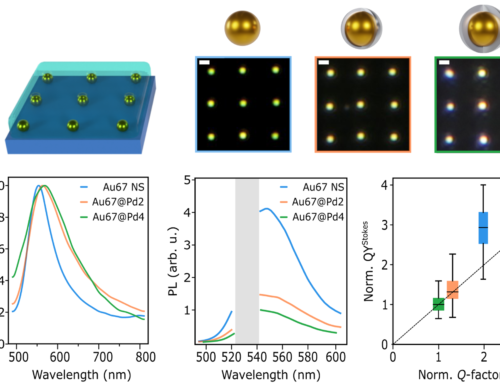
a) Examples of super-resolution imaging of DNA origami samples obtained through DNA-PAINT measurements on a confocal microscope. b) Histogram plot of the localizations obtained with the confocal (left) and the widefield (right) microscopes. For the comparison, the same area size and number of localizations were employed. c) Schematic of the measurement pipeline for super-resolved FLIM. Raw confocal images are binned to obtain a frame for SMLM. Next, for each single molecule emission event used for a localization, photon arrival times are analysed to obtain the excited state lifetime. d) Distribution of lifetimes of all localizations in a particular FoV. The total distributions of lifetimes is well represented by a sum of two Gaussian distributions (black line), one ascribed to the D in the absence of FRET (magenta) and the other to A and D undergoing FRET (cyan). A threshold of 3.2 ns was used to differentiate single-molecule emission presenting FRET (lifetime > 3.2 ns) or no FRET (lifetime < 3.2 ns). e) Super-resolved FLIM of a selection of DNA origami, using a binary lifetime color code according to (d), and with the intensity proportional to the number of localizations. Scale bars 200 nm.
Read the full article:
Cecilia Zaza, Germán Chiarelli, Ludovit P. Zweifel, Mauricio Pilo-Pais, Evangelos Sisamakis, Fernando D. Stefani, Guillermo P. Acuna